Which compounds have been withdrawn from the market?
Reference




OpenAI's Answer
The withdrawal of compounds from the market generally occurs due to safety concerns or ineffectiveness. Here are some notable examples:
- Thalidomide: Withdrawn in the 1960s due to severe birth defects.
- Ranitidine (Zantac): Withdrawn due to contamination with NDMA, a probable carcinogen.
- Melflufen: A derivative of melphalan, was withdrawn after excess deaths in a phase three confirmatory trial.
- Vioxx (Rofecoxib): Withdrawn due to increased risk of heart attack and stroke.
- Fen-Phen (Fenfluramine/Phentermine): Withdrawn because of heart valve disease and pulmonary hypertension.
- Sibutramine (Meridia): Withdrawn due to increased risk of heart attack and stroke.
- Bextra (Valdecoxib): Withdrawn due to cardiovascular risks and severe skin reactions.
- Tegaserod (Zelnorm): Withdrawn due to increased risk of heart attack and stroke.
- Cisapride (Propulsid): Withdrawn due to serious cardiac side effects.
For a comprehensive list and further details, you can check resources such as the Federal Register and the FDA's official listings.
Images:
- Examples of drugs withdrawn due to safety issues:

- Workflow and examples of recalled pharmaceutical compounds:
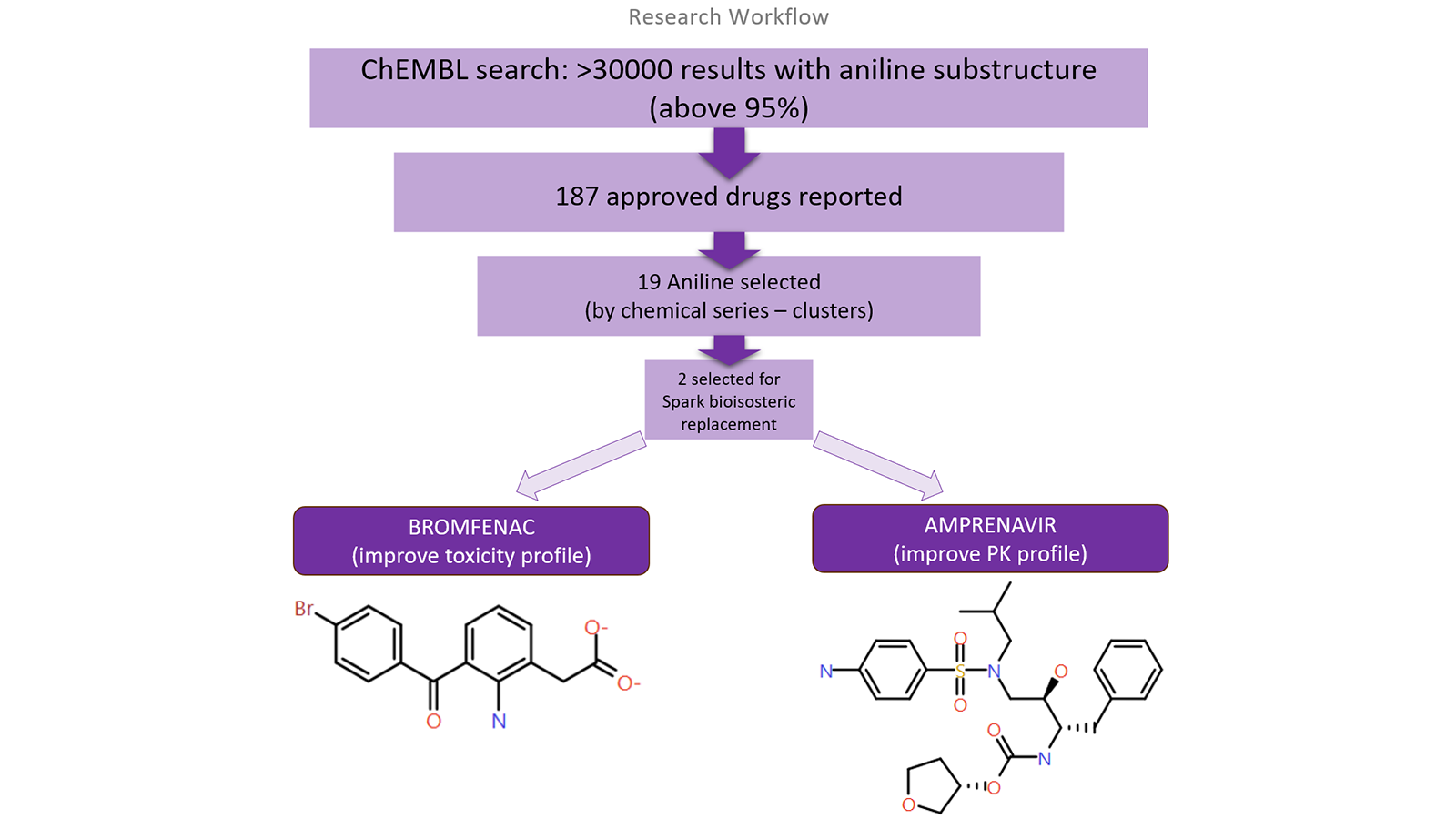
These examples highlight the rigorous monitoring and proactive steps taken to ensure drug safety and efficacy post-market approval.
Follow Up
Related
What are volatile organic compounds, and why are they regulated in paints?
What can the presence of hydrocarbons in comets tell us about the origins of organic compounds in sp
What are the key differences between simple and compound attacks in fencing?
How do trimethylsilyl groups affect the volatility of compounds in gas chromatography?