Reference

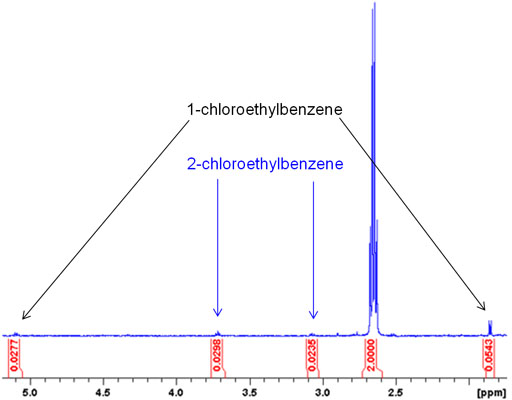
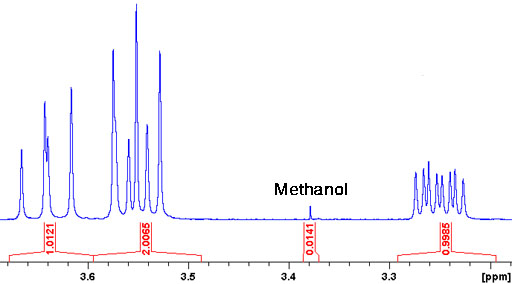

OpenAI's Answer
In NMR spectroscopy, common laboratory solvents can contain various impurities that may interfere with the spectra. Here are some common impurities detected in NMR spectroscopy:
-
Acetone (C3H6O):
- 1H NMR: Singlet at δ 2.17 ppm
- 13C NMR: Singlet at δ 206.7 ppm
-
Chloroform (CHCl3):
- 1H NMR: Singlet at δ 7.26 ppm
- 13C NMR: Singlet at δ 77.23 ppm
-
Methanol (CH3OH):
- 1H NMR: Singlet at δ 3.31 ppm
- 13C NMR: Singlet at δ 49.50 ppm
-
Diethyl Ether (C4H10O):
- 1H NMR: Triplets at δ 1.21 and 3.45 ppm
- 13C NMR: Singlets at δ 15.18 and 66.53 ppm
-
Tetrahydrofuran (THF, C4H8O):
- 1H NMR: Multiplets at δ 1.73 and 3.58 ppm
- 13C NMR: Singlets at δ 25.31 and 67.21 ppm
These impurities are common because they are often used as solvents or reagents in various chemical processes. Their NMR signals can be distinguished by their unique chemical shifts in both proton (^1H) and carbon (^13C) NMR spectroscopy.
References:
- NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents
- NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities
If you'd like, here are some of the relevant images related to impurities detected in NMR spectroscopy:
By understanding these impurities, chemists can better interpret their NMR results and account for these background signals.