Reference


OpenAI's Answer
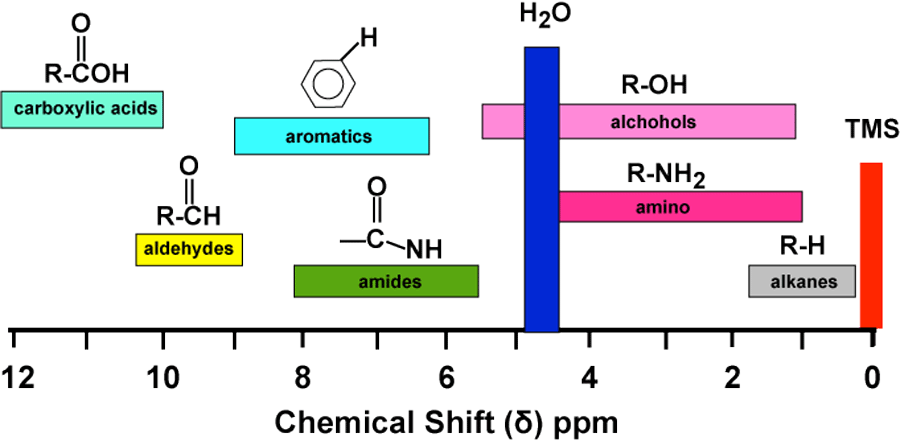
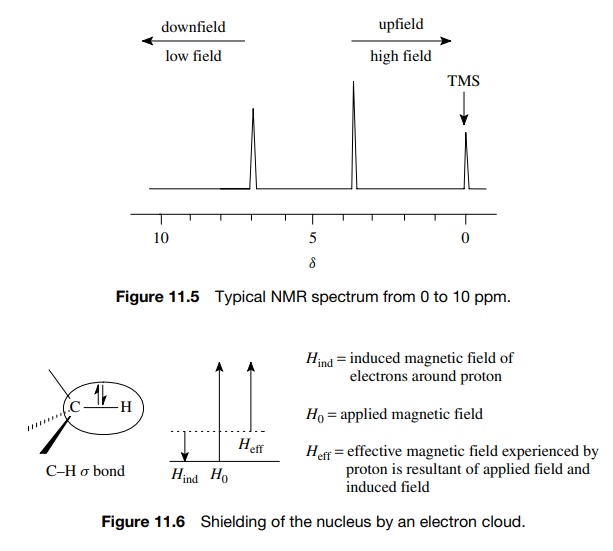
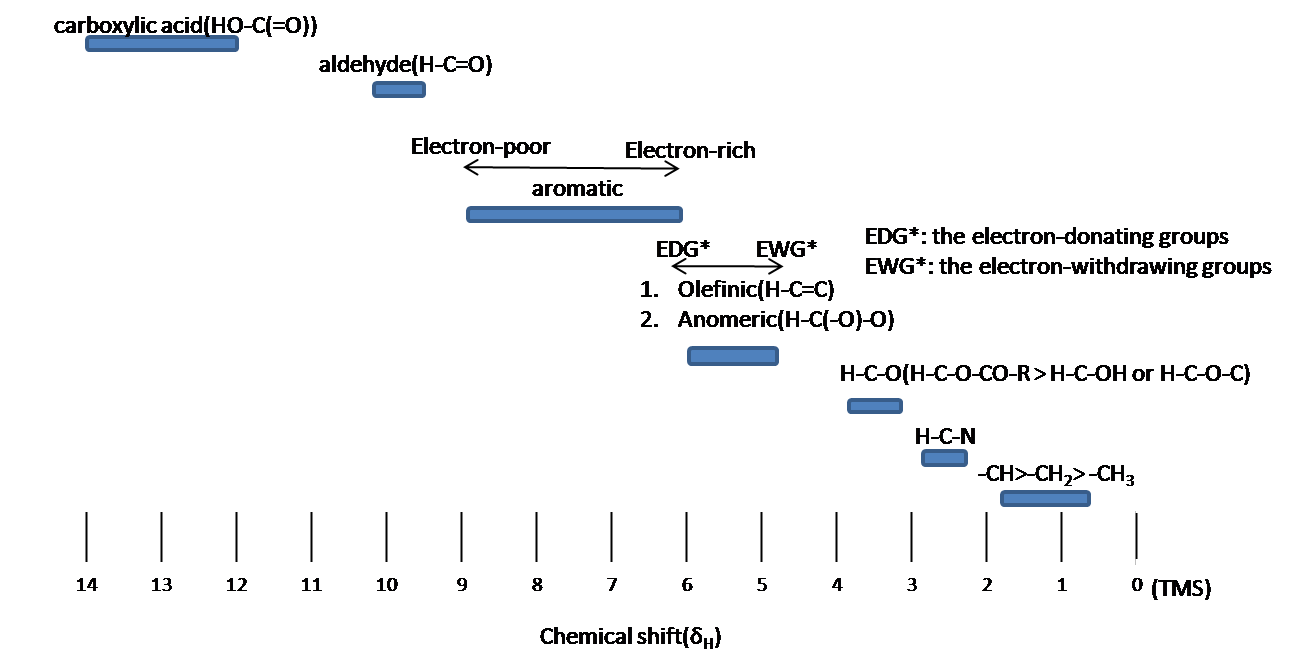
In NMR spectroscopy, the chemical shift of trimethylsilyl (TMS) groups is significant due to several reasons:
-
Reference Standard: TMS, specifically tetramethylsilane (Si(CH₃)₄), is universally used as an internal standard for calibrating the chemical shift scale in both proton (¹H) and carbon (¹³C) NMR spectroscopy. Its signal is set to 0 ppm, providing a reference point against which all other chemical shifts are measured (Chemistry LibreTexts).
-
Chemical Inertness: TMS is chemically inert and does not react with most solvents or samples, making it an ideal reference compound. This inertness ensures that it does not interfere with the sample being analyzed.
-
Sharp Signal: The twelve protons of TMS, all equivalent, produce a single, strong, and sharp signal, which enhances the precision and accuracy of chemical shift measurements (ScienceDirect).
-
Universal Solubility: TMS is soluble in most organic solvents. This wide solubility range ensures it can be used in a variety of NMR experiments across different solvent systems without issues.
-
Non-Interference: The signal of TMS falls outside the range of most organic compounds' signals in the ¹H NMR spectrum, reducing the likelihood of peak overlap and ensuring clear interpretable data (Chemistry LibreTexts).
These features make the chemical shift of trimethylsilyl groups a crucial aspect in NMR spectroscopy, supporting accurate and reliable spectral analysis. Below is an image summarizing TMS's role in NMR:
For further details, you can access the original resources: