Reference


Reference



OpenAI's Answer
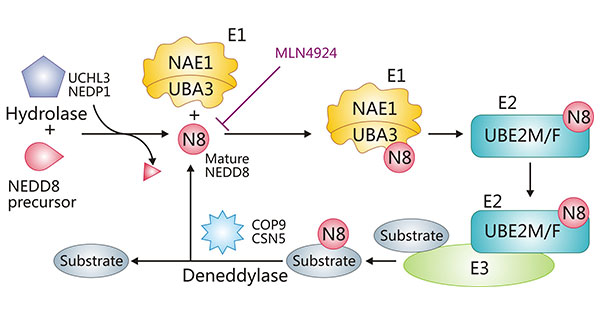
In the NEDD8 conjugation pathway, UBA3 plays a crucial role as part of the E1 enzyme complex. UBA3 is the catalytic subunit of the NEDD8-activating enzyme E1, which is a dimeric enzyme complex involving APPBP1 (also known as NAE1). This enzyme complex is responsible for the activation of the NEDD8 protein. Specifically, UBA3 catalyzes the adenylation of the C-terminal glycine residue of NEDD8 using ATP, which is the first step in the conjugation of NEDD8 to substrate proteins. This adenylation facilitates the transfer of NEDD8 to a cysteine residue in the UBA3 subunit, effectively leading to the "activation" of NEDD8 (source, source).
The NEDD8 conjugation pathway is vital for regulating various cellular processes, including cell cycle progression and protein degradation. This pathway is similar to ubiquitination but involves the neddylation of specific substrates like cullins, which are components of cullin-RING ligases (CRLs) that target proteins for degradation (source).
Regarding SAPE1, there seems to be no direct information available in the current search results about a role it might play in the NEDD8 conjugation pathway. It could be possible that SAPE1 is either less studied or its involvement isn't as prominent in the literature accessible at the moment.
Images depicting the NEDD8 conjugation and related processes can be found on ResearchGate and Cusabio.
Follow Up
Related
What is the significance of NEDD8 conjugation to proteins in cellular mechanisms?
What are the primary tissues where UBE2M is expressed in humans and mice?
How does the dominant-negative UBC12 mutant affect NEDD8 conjugation in vivo?
What role does the ubiquitin-like protein NEDD8 play in the ubiquitin-proteasome system?